
Δρ.
Μάτσα Ρεβέκκα
Συνεργαζόμενη Ερευνήτρια
Επιστημονικώς Υπεύθυνη Ερευνητικών Έργων και Προγραμμάτων
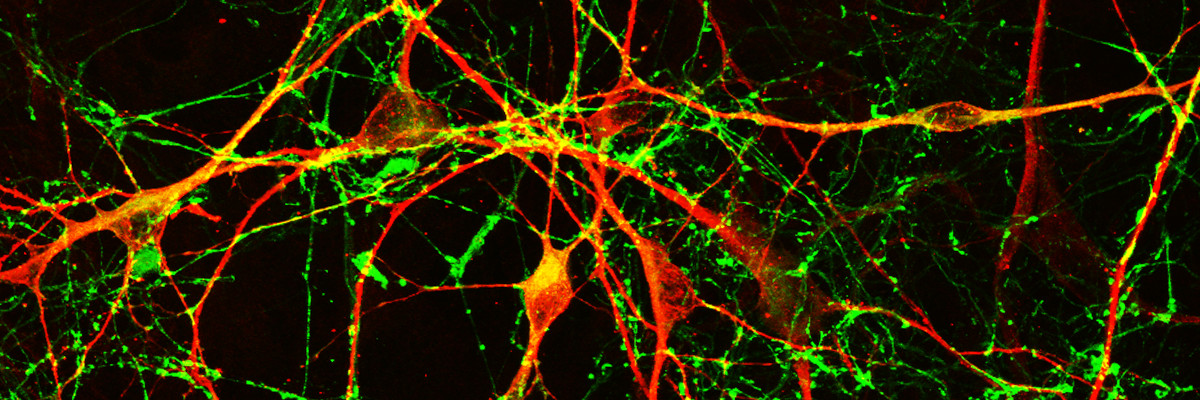
Our current research aims at studying human brain development and disease in conditions such as Parkinson’s disease (PD). To this end, we generate models with human induced pluripotent stem cells to investigate a) the development and evolution of human brain and b) mechanisms of neurodegeneration in familial PD.
Understanding the mechanisms underlying human brain development and disease has long been hampered by limited access to the living brain and the inability to isolate and maintain live human neurons. The advent of human stem cells in combination with cellular reprogramming technologies has opened new perspectives in the field of brain research. Human embryonic stem cells and induced pluripotent stem cells (iPSCs) generated by reprogramming of differentiated somatic cells to pluripotency, provide for the first time the opportunity to foster live human neurons and glial cells in the laboratory.
- Patient-derived model for Parkinson’s disease pathogenesis: a platform for mechanistic studies and the identification of disease-modifying compounds
A major focus of research has been the generation of iPSCs from skin fibroblasts of patients with familial Parkinson’s disease bearing the p.A53T mutation in the α-synuclein protein (G209A in the SNCA gene) to elucidate disease mechanisms and identify new therapeutic targets. Directing their differentiation to neurons, we developed a 2-D clinically relevant model displaying distinct disease phenotypes that has been an instrumental biological system for mechanistic studies of disease pathogenesis and drug discovery (Kouroupi et al PNAS 2017; Taoufik et al Open Biol 2018; Grudina, Kouroupi et al Neurobiol Dis 2019; Elkouris, Kouroupi et al Front Cell Neurosci. 2019 ; Kouroupi et al Int J Mol Sci 2020; Int J Dev Biol. 2021; Kim… Kouroupi et al Front Microbiol. 2021; Antoniou et al npj Parkinson’s Disease 2022).
Recently, we received funding from the Hellenic Foundation for Research and Innovation to develop a research project on the contribution of astrocytes in p.A53T pathology and investigate neuron-glia dynamics in Parkinson’s disease.
- High Content imaging and compound library screening identifies novel therapeutics for Parkinson’s disease
The p.A53T system has been adapted for high-content imaging and compound library screening, which identified the multi-kinase inhibitor BX795 as a promising neuroprotective molecule and candidate therapeutic for PD and possibly other protein conformational disorders. Proteomics profiling mapped the molecular pathways underlying the protective effects of BX795, comprising a cohort of 118 protein-mediators of the core biological processes of RNA metabolism, protein synthesis, modification and clearance, and stress response, all linked to the mTORC1 signaling hub. In agreement, expression of human p.A53T-αSyn in neuronal cells affected key components of the mTORC1 pathway resulting in aberrant protein synthesis that was restored in the presence of BX795 with concurrent facilitation of autophagy (Antoniou et al 2022 npj Parkinson’s disease). Research is ongoing to identify BX795 direct targets.
- Uptake and transmission of pathological proteins implicated in Parkinson’s disease
We collaborate with Prof. Chiara Zurzolo from the Institut Pasteur in Paris within the framework of a Pasteur International Unit (PIU) on Neurodegenerative diseases. Within this framework and in collaboration with researchers at the Tokyo Metropolitan Institute of Medical Science we investigated the uptake and transmission of α-synuclein implicated in Parkinson’s disease pathology. Using our human p.A53T-iPSC derived model, we found that the transfer of α-synuclein fibrils is mediated primarily by a novel cell contact mechanism through tunneling nanotubes, indicating that the ability of neural cells to uptake and transfer pathological proteins may limit their effectiveness in therapeutic transplantation for treatment of neurodegenerative diseases (Grudina, Kouroupi et al Neurobiol Dis 2019).
- In vivo modeling of p.A53T-aSyn pathology
We investigated the in vivo fate and phenotypes of patient-derived p.A53T-iPSC derived neurons after transplantation in a chemically-induced mouse model of Parkinson’s disease established by unilateral intrastriatal 6-hydroxydopamine injection in the immunosuppressed NOD/SCID strain. We found that mutant cells could survive and differentiate in vivo over a 12-week period. However, at this time point they displayed significantly increased levels of α-synuclein, signaling a first step towards pathology, and showed compromised integration within the host tissue. This data imply caution regarding the long-term consequences of autologous transplantation as a therapeutic strategy for Parkinson’s disease patients (Zygogianni et al Methods Mol Biol. 2020; Neurochem Res. 2019).
- Development of a human stem cell-based model of human brain development: investigation of human-associated traits of neurodevelopment
An iPSC-based system has been generated for modeling human brain development in 2D cultures. Integrated mRNA/miRNA transcriptome analysis at distinct stages of directed neuronal differentiation identified miR-934, a human-associated miRNA that displays a stage-specific expression pattern during progenitor expansion and early neuron generation. We demonstrated the biological relevance of this finding by comparison with data from early to mid-gestation human cortical tissue. miR-934 directly controls progenitor to neuroblast transition and impacts on neurite growth of newborn neurons. Its inhibition results in profound global transcriptome changes associated with neurogenesis while affecting the expression of genes associated with the subplate zone, a transient compartment most prominent in primates that emerges during early corticogenesis. Our work highlights mir-934 as a novel regulator of early human neurogenesis with potential implications for a species-specific evolutionary role in brain function (Prodromidou and Matsas Cell Mol Life Sci 2021; Prodromidou et al. eLife 2020; Prodromidou and Matsas Front Cell Neurosci. 2019).
This new core facility initiative, aims to provide capacity for high-throughput high-content imaging and phenotypic screening, using cellular disease models and tissue organoids for investigation of poorly understood complex human conditions, such as neurodegenerative diseases.
Recent advances in cell reprogramming technologies have facilitated the generation of patient-derived models for neurodegenerative diseases and the identification of early, potentially triggering, pathological phenotypes while they provide amenable systems for drug discovery.
The Platform aims at combining disease-modeling, automated high-content imaging, compound library screening and multi-electrode array electrophysiological recordings for integrated morphological, molecular, cellular and functional studies.
Setting-off in relevance to neurodegenerative diseases, the platform aims to provide expertise for collaborative ventures and services to the scientific community, and expand its activities in infectious and chronic diseases.
Platform development is supported by the Hellenic Foundation for Research & Innovation (HFRI) ELIDEK; 1st Call for H.F.R.I. Research Projects to Support Faculty Members & Researchers and the Procurement of High-Value Research Equipment (H.F.R.I grant 1019-DiseasePhenoTarget to R. Matsas).
Hellenic Foundation for Research and Innovation (HFRI) 2021-2025: Development of a multiparametric morphofunctional platform to uncover disease mechanisms and druggable targets in patient derived cells: study of neuron-glia dynamics in alpha synuclein-mediated pathologies» (1019-DiseasePHENOTarget)
Hellenic General Secretariat for Research and Innovation (GSRI) Operational Programme Competitiveness, Entrepreneurship and Innovation 2014-2020 (EPAnEK – NSRF 2014-2020), 2021-2023: Systematic development and commercial exploitation of novel compounds inhibiting alpha-synuclein aggregation ( AlphaSyn MIS 5131418)
Creation of a Virtual Research Unit for Studies in Neurodegenerative Diseases 2022-2025. PIs Rebecca Matsas (Hellenic Pasteur Institute) and Chiara Zurzolo (Institut Pasteur-Paris).
Hellenic General Secretariat for Research and Innovation (GSRI) –NSFR 2014-2020 Flagship Action for Neurodegenerative Diseases on the basis of Personalized Medicine (2020-2023)
Priority Axis «Strengthening the Mechanisms and Investments of SMEs of the Attica Region in Research and Innovation» funded by the Operational Program «Attiki 2014-2020» (NSFR 2014-2020) «High Technology Infrastructure for Preclinical Studies and Provision of Specialized Services for Infectious and Neurodegenerative Diseases» 2021-2023 (MIS 5066768)
Institut Pasteur Direction du Développement GRAND PROGRAMME FÉDÉRATEUR M&B MICROBES & BRAIN PROJECTS 2016: SEEDS FOR INNOVATION 2017-2018: In vivo models of brain infection by Gram+ bacteria: from barrier crossing to neural stem cell (InFeSteR).
Hellenic Foundation for Research and Innovation (HFRI) 2018-2021: Mechanisms of synaptic dysfunction in human induced pluripotent stem cell-based 2D- and 3D-models of familial Parkinson’s disease (PARKINSynapse).
Stavros Niarchos Foundation Grant 2016-2020: Development of innovative biological products and services for infectious and neurodegenerative diseases Coordinated by R. Matsas and V. Miriagou
Institut Pasteur Transversal Research Program 2015-2018 PTR-523: Mechanisms of pathological A53T-alpha-synuclein transmission in human iPS-derived neurons. Coordinated by R. Matsas; Participants: C. Zurzolo, PM Lledo and F. Lazarini IP-Paris
Fondation Santé Grant 2014-2016: An integrated genome-wide miRNA-mRNA approach in a human stem cell-based model of neurodevelopment and disease.
Greek Ministry of Education 2012-2015: EXCELLENCE GRANT I-2272 - Modeling Parkinson’s disease by conversion of fibroblasts to dopaminergic neurons.
Greek General Secretariat for Research and Technology 2011-2015: Cooperation GRANT 09SYN-21–969 - Mechanisms of Induced Pluripotency: From Transcriptional Noise to Stem Cell Therapies.
Greek General Secretariat for Research and Technology 2013-2015 KRHPIS GRANT InfeNeuTra - MIS450598: Infectious and Neurodegenerative diseases: From study of basic mechanisms to development of translational research towards prevention and therapy.
Greek General Secretariat for Research and Technology 2013-2015: THALES GRANT KA3578 “Mitochondrial Dysfunction in Neurodegenerative Diseases”
Institut Pasteur Transversal Research Program Grant 2013-2015 PTR-417: Parkinsonian patient-derived dopaminergic neurons for disease modeling and drug discovery. Coordinated by R. Matsas; Participants: D. Bohl IP-Paris, R. Grailhe IP-Korea.
Empeirikion Foundation 2013-2014 Generation of patient-derived induced pluripotent stem cells
Fondation BNP Paribas 2010-2013: Use of human stem cells for the treatment of neurodegenerative diseases and injuries of the brain and spinal cord.
EU FP7 Program Grant 264083 NEUROSIGN 2010-2013: Development of a Centre of Excellence in Neurosignalling (Tzartos, Matsas and Probert).
Bodossaki Foundation Award, 2009-2010: Establishment of a Stem Cell and Transplantation Facility.
Wings for Life Foundation for Spinal Cord Research 2006-2007: Therapeutic potential of Schwann cells genetically engineered to express PSA after transplantation in the lesioned mouse spinal cord.
Greek General Secretariat for Research and Technology 2005-08: Development of infrastructure in cutting-edge technologies for innovative diagnostic and therapeutic strategies. (Matsas, Mamalaki, Probert and Soteriadou)
Greek General Secretariat for Research and Technology Programme PENED, 2005-2007. Application of the neuroprotective factor IGF1 for the treatment of brain trauma and neurodegeneration
Greek General Secretariat for Research and Technology Programme EPAN, 2004-2006 Neural Stem Cell Therapies for Neurodegenerative Diseases: Determination of a “molecular signature” for neuronal fate, YB26:
Greek General Secretariat for Research and Technology Programme EPAN, 2004-2006. Baculovirus Artificial Chromosomes (BVACs) and Technologies for Gene Therapy and Continuous High-Level Expression of Therapeutic Proteins in Insect Production Systems, YB11.
EU Training and Mobility Programme – Neurosciences, 2003-2005. Role of calcium mobilization in neuronal differentiation: novel aspects of single cell and organellar calcium signals. QLG2-CT-2002-51680
EU Quality of Life Programme – Neurosciences, 2000-2003. Genes controlling neuronal specification and differentiation QLG3-CT-00072
EU Quality of Life Programme – Neurosciences, 2000-2003 Engineering neural precursors for myelin repair. QLG3-CT-00911
Full list of publications in PUBMED
2021-2
Nasia Antoniou, Kanella Prodromidou, Georgia Kouroupi, Ioanna Boumpoureka, Martina Samiotaki, George Panayotou, Maria Xilouri, Ismini Kloukina, Leonidas Stefanis, Regis Grailhe, Era Taoufik and Rebecca Matsas. High Content Screening and Proteomic Analysis Identify a Kinase Inhibitor that rescues pathological phenotypes in a Patient-Derived Model of Parkinson's Disease. npj Parkinson’s Disease 2022 Feb 11;8:15 ; doi: 10.1038/s41531-022-00278-y.
Akrioti E, Karamitros T, Gkaravelas P, Kouroupi G, Matsas R, Taoufik E. Early Signs of Molecular Defects in iPSC-Derived Neural Stems Cells from Patients with Familial Parkinson's Disease. Biomolecules. 2022 Jun 23;12(7):876. doi: 10.3390/biom12070876.
Kanella Prodromidou and R. Matsas. Evolving features of human cortical development and the emerging roles of non-coding RNAs in neural progenitor cell diversity and function. Cell Mol Life Sci 2021, Dec 18;79(1):56. doi: 10.1007/s00018-021-04063-7.
Seonhee Kim, Florence Larrous, Hugo Varet, Rachel Legendre, Lena Feige, Guillaume Dumas, Rebecca Matsas, Georgia Kouroupi, Regis Grailhe and Hervé Bourhy. Early transcriptional changes in rabies virus-infected neurons and their impact on neuronal functions Frontiers in Microbiology 2021, Dec 13;12:730892. doi: 10.3389/fmicb.2021.730892.
Georgia Kouroupi, Kanella Prodromidou, Florentia Papastefanaki, Era Taoufik and Rebecca Matsas Organoids: the third dimension of human brain development and disease. Int J Dev Biol 2021 Oct 26 doi: 10.1387/ijdb.210158gk. (Contribution by invitation to a Special Issue devoted to Developmental Biology in Greece)
Mladenovic Djordjevic AN, Kapetanou M, Loncarevic-Vasiljkovic N, Todorovic S, Athanasopoulou S, Jovic M, Prvulovic M, Taoufik E, Matsas R, Kanazir S, Gonos ES. Pharmacological intervention in a transgenic mouse model improves Alzheimer's-associated pathological phenotype: Involvement of proteasome activation. Free Radic Biol Med. 2021 Jan; 162:88-103. doi: 10.1016/j.freeradbiomed.2020.11.038.
2020
Benmimoun B, Papastefanaki F, Périchon B, Segklia K, Roby N, Miriagou V, Schmitt C, Dramsi S, Matsas R, Spéder P. An original infection model identifies host lipoprotein import as a route for blood-brain barrier crossing. Nat Commun. 2020 Nov 30;11(1):6106. doi: 10.1038/s41467-020-19826-2.
Kouroupi G, Antoniou N, Prodromidou K, Taoufik E, Matsas R Patient-Derived Induced Pluripotent Stem Cell-Based Models in Parkinson's Disease for Drug Identification. Int J Mol Sci. 2020 Sep 26;21(19):7113. doi: 10.3390/ijms21197113.
Zygogianni O, Kouroupi G, Taoufik E, Matsas R. Engraftable Induced Pluripotent Stem Cell-Derived Neural Precursors for Brain Repair. Methods Mol Biol. 2020;2155:23-39. doi: 10.1007/978-1-0716-0655-1_3.
Prodromidou K, Vlachos IS, Gaitanou M, Kouroupi G, Hatzigeorgiou AG, Matsas R. MicroRNA-934 is a novel primate-specific small non-coding RNA with neurogenic function during early development. Elife. 2020 May 27;9:e50561. doi: 10.7554/eLife.50561.
2019
Prodromidou K, Matsas R. Species-Specific miRNAs in Human Brain Development and Disease. Front Cell Neurosci. 2019 Dec 18;13:559. doi: 10.3389/fncel.2019.00559. eCollection 2019.
Grudina C, Kouroupi G, Nonaka T, Hasegawa M, Matsas R, Zurzolo C. Human NPCs can degrade α-syn fibrils and transfer them preferentially in a cell contact-dependent manner possibly through TNT-like structures. Neurobiol Dis. 2019 Dec;132:104609. doi: 10.1016/j.nbd.2019.104609. Epub 2019 Sep 5.
Gaitanou M, Segklia K, Matsas R. Cend1, a Story with Many Tales: From Regulation of Cell Cycle Progression/Exit of Neural Stem Cells to Brain Structure and Function. Stem Cells Int. 2019 May 2;2019:2054783. doi: 10.1155/2019/2054783. eCollection 2019.
Tzortzopoulos A, Thomaidou D, Gaitanou M, Matsas R, Skoulakis E. Expression of Mammalian BM88/CEND1 in Drosophila Affects Nervous System Development by Interfering with Precursor Cell Formation. Neurosci Bull. 2019 Dec;35(6):979-995. doi: 10.1007/s12264-019-00386-5. Epub 2019 May 11.
Zygogianni O, Antoniou N, Kalomoiri M, Kouroupi G, Taoufik E, Matsas R.In Vivo Phenotyping of Familial Parkinson's Disease with Human Induced Pluripotent Stem Cells: A Proof-of-Concept Study. Neurochem Res. 2019 Jun;44(6):1475-1493. doi: 10.1007/s11064-019-02781-w. Epub 2019 Apr 15.
Elkouris M, Kouroupi G, Vourvoukelis A, Papagiannakis N, Kaltezioti V, Matsas R, Stefanis L, Xilouri M, Politis PK. Long Non-coding RNAs Associated With Neurodegeneration-Linked Genes Are Reduced in Parkinson's Disease Patients. Front Cell Neurosci. 2019 Feb 22;13:58. doi: 10.3389/fncel.2019.00058. eCollection 2019.
Segklia K, Stamatakis A, Stylianopoulou F, Lavdas AA, Matsas R. Increased Anxiety-Related Behavior, Impaired Cognitive Function and Cellular Alterations in the Brain of Cend1-deficient Mice. Front Cell Neurosci. 2019 Jan 29;12:497. doi: 10.3389/fncel.2018.00497. eCollection 2018.
2017-8
Taoufik E, Kouroupi G, Zygogianni O, Matsas R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: an overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018 Sep;8(9):180138. doi: 10.1098/rsob.180138.
Kouroupi G, Taoufik E, Vlachos IS, Tsioras K, Antoniou N, Papastefanaki F, Chroni-Tzartou D, Wrasidlo W, Bohl D, Stellas D, Politis PK, Vekrellis K, Papadimitriou D, Stefanis L, Bregestovski P, Hatzigeorgiou AG, Masliah E, Matsas R. Defective synaptic connectivity and axonal neuropathology in a human iPSC-based model of familial Parkinson's disease. Proc Natl Acad Sci U S A. 2017 May 2;114(18):E3679-E3688. doi: 10.1073/pnas.1617259114. Epub 2017 Apr 17.
Terzenidou ME, Segklia A, Kano T, Papastefanaki F, Karakostas A, Charalambous M, Ioakeimidis F, Papadaki M, Kloukina I, Chrysanthou-Piterou M, Samiotaki M, Panayotou G, Matsas R, Douni E. Novel insights into SLC25A46-related pathologies in a genetic mouse model. PLoS Genet. 2017 Apr 4;13(4):e1006656. doi: 10.1371/journal.pgen.1006656. eCollection 2017 Apr.
2015-16
Koutsoudaki PN, Papastefanaki F, Stamatakis A, Kouroupi G, Xingi E, Stylianopoulou F, Matsas R. Neural stem/progenitor cells differentiate into oligodendrocytes, reduce inflammation, and ameliorate learning deficits after transplantation in a mouse model of traumatic brain injury. Glia. 2016 May;64(5):763-79. doi: 10.1002/glia.22959. Epub 2015 Dec 29.
Aravantinou-Fatorou K, Ortega F, Chroni-Tzartou D, Antoniou N, Poulopoulou C, Politis PK, Berninger B, Matsas R, Thomaidou D. CEND1 and NEUROGENIN2 Reprogram Mouse Astrocytes and Embryonic Fibroblasts to Induced Neural Precursors and Differentiated Neurons. Stem Cell Reports 2015 Sep 8;5(3):405-18. doi: 10.1016/j.stemcr.2015.07.012. Epub 2015 Aug 28.
Papastefanaki F, Jakovcevski I, Poulia N, Djogo N, Schulz F, Martinovic T, Ciric D, Loers G, Vossmeyer T, Weller H, Schachner M, Matsas R. Intraspinal Delivery of Polyethylene Glycol-coated Gold Nanoparticles Promotes Functional Recovery After Spinal Cord Injury. Mol Ther. 2015 Jun;23(6):993-1002. doi: 10.1038/mt.2015.50. Epub 2015 Mar 25.
Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015 Jul;63(7):1101-25. doi: 10.1002/glia.22809. Epub 2015 Mar 2.
2011-14
Prodromidou K, Papastefanaki F, Sklaviadis T, Matsas R. Functional cross-talk between the cellular prion protein and the neural cell adhesion molecule is critical for neuronal differentiation of neural stem/precursor cells. Stem Cells. 2014 Jun;32(6):1674-87. doi: 10.1002/stem.1663.
Tsioras K, Papastefanaki F, Politis PK, Matsas R, Gaitanou M. Functional Interactions between BM88/Cend1, Ran-binding protein M and Dyrk1B kinase affect cyclin D1 levels and cell cycle progression/exit in mouse neuroblastoma cells. PLoS One. 2013 Nov 28;8(11):e82172. doi: 10.1371/journal.pone.0082172. eCollection 2013.
Miltiadous P, Kouroupi G, Stamatakis A, Koutsoudaki PN, Matsas R, Stylianopoulou F. Subventricular zone-derived neural stem cell grafts protect against hippocampal degeneration and restore cognitive function in the mouse following intrahippocampal kainic acid administration. Stem Cells Transl Med. 2013 Mar;2(3):185-98. doi: 10.5966/sctm.2012-0074. Epub 2013 Feb 15.
Ziavra D, Makri G, Giompres P, Taraviras S, Thomaidou D, Matsas R, Mitsacos A, Kouvelas ED. Neural stem cells transplanted in a mouse model of Parkinson's disease differentiate to neuronal phenotypes and reduce rotational deficit. CNS Neurol Disord Drug Targets. 2012 Nov 1;11(7):829-35. doi: 10.2174/1871527311201070829.
Lavdas AA, Papastefanaki F, Thomaidou D, Matsas R. Cell adhesion molecules in gene and cell therapy approaches for nervous system repair. Curr Gene Ther. 2011 Apr;11(2):90-100. doi: 10.2174/156652311794940755.
2010
Kaltezioti V, Kouroupi G, Oikonomaki M, Mantouvalou E, Charonis A, Rohrer H, Matsas R and Politis PK (2010) Prox1 suppresses Notch1 gene expression to regulate neurogenesis in the spinal cord, PLOS Biology, in revision.
Lavdas AA, Papastefanaki F, Thomaidou D and Matsas R (2010) Cell adhesion molecules in gene and cell therapy approaches for nervous system repair Curr Gene Ther, invited review.
Kouroupi G, Lavdas AA, Gaitanou M, Thomaidou D, Stylianopoulou F and Matsas R (2010) Lentivirus-mediated expression of insulin-like growth factor-I promotes neural stem/precursor cell proliferation and enhances their potential to generate neurons J Neurochem, accepted for publication.
Lavdas AA, Efrose R, Douris V, Gaitanou M, Swevers L, Thomaidou D, Iatrou K and Matsas R (2010) Soluble forms of the cell adhesion molecule L1 produced by insect and baculovirus-transduced mammalian cells enhance Schwann cell motility J Neurochem, accepted for publication.
Sergaki MC, Guillemot F and Matsas R (2010) Impaired cerebellar development and deficits in motor coordination in mice lacking the neuronal protein BM88/Cend1. Mol Cell Neurosci, 44(1):15-29.
Makri G, Lavdas AA, Katsimpardi L, Charneau P, Thomaidou D and Matsas R (2010) Transplantation of embryonic neural stem/ precursor cells overexpressing BM88/Cend1 enhances the generation of neuronal cells in the injured mouse cortex. Stem Cells 28(1):127-39. Epub Nov 2009.
Lavdas AA, Chen J, Papastefanaki F, Chen S, Schachner M, Matsas R and Thomaidou D(2010) Schwann cells engineered to express the cell adhesion molecule L1 accelerate myelination and motor recovery after spinal cord injury. Exp Neurol 221(1):206-16. Epub Nov 2009.
2009
Masgrau R, Hurel C, Papastefanaki F, Georgopoulou N, Thomaidou D and Matsas R (2009) BM88/Cend1 regulates stimuli-induced calcium mobilization. Neuropharmacology, 56:598-609.
Lavdas, AA and Matsas, R (2009) Towards personalized cell-replacement therapies for brain repair Personalized Medicine 6(3): 293-313.
2008
Katsimpardi L, M Gaitanou, CE Malnou, PM Lledo, P. Charneau, R Matsas and D Thomaidou (2008) BM88/Cend1 expression levels are critical for proliferation and differentiation of subventricular zone-derived neural precursor cells. Stem Cells 26:1796-807.
Politis PK, Akrivou S, Hurel C, Papadodima O, Matsas R. (2008) BM88/Cend1 is involved in histone deacetylase inhibition-mediated growth arrest and differentiation of neuroblastoma cells. FEBS Lett. 582:741-8.
Politis PK, Thomaidou D, Matsas R. (2008) Coordination of cell cycle exit and differentiation of neuronal progenitors. Cell Cycle Mar 15;7(6):691-7.
Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E. (2008) A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem. 283:2031-41.
Lavdas AA, Papastefanaki F, Thomaidou D, Matsas R (2008) Schwann cell transplantation for CNS repair. Curr Med Chem. 15:151-60.
2007
Politis PK, Makri G, Thomaidou D, Geissen M, Rohrer H, Matsas R. (2007) BM88/CEND1 coordinates cell cycle exit and differentiation of neuronal precursors. Proc Natl Acad Sci U S A. 2104 (45):17861-6.
Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. (2007) Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 130:2159-74.
Politis PK, Rohrer H, Matsas R. (2007) Expression pattern of BM88 in the developing nervous system of the chick and mouse embryo. Gene Expr Patterns 7:165-77.
Gravvanis AI, Lavdas AA, Papalois A, Tsoutsos DA, Matsas R. (2007) The beneficial effect of genetically engineered Schwann cells with enhanced motility in peripheral nerve regeneration Acta Neurochir Suppl.;100:51-6.
2006
Lavdas AA, Franceschini I, Dubois-Dalcq M, Matsas R. (2006) Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia 53(8):868-78.
Kenoutis C, Efrose RC, Swevers L, Lavdas AA, Gaitanou M, Matsas R, Iatrou K. (2006) Baculovirus-mediated gene delivery into Mammalian cells does not alter their transcriptional and differentiating potential but is accompanied by early viral gene expression. J Virol. 80:4135-46.
Georgopoulou N, Hurel C, Politis PK, Gaitanou M, Matsas R, Thomaidou D. (2006) BM88 is a dual function molecule inducing cell cycle exit and neuronal differentiation of neuroblastoma cells via cyclin D1 down-regulation and retinoblastoma protein hypophosphorylation. J Biol Chem. 281:33606-20.
2005
Papadodima O, Sergaki M, Hurel C, Mamalaki A, Matsas R. (2005) Characterization of the BM88 promoter and identification of an 88 bp fragment sufficient to drive neurone-specific expression. J Neurochem. 95:146-59.
Gravvanis AI, Lavdas A, Papalois AE, Franceschini I, Tsoutsos DA, Dubois-Dalcq M, Matsas R, Ioannovich JD. (2005) Effect of genetically modified Schwann cells with increased motility in end-to-side nerve grafting. Microsurgery 2005;25(5):423-32.
Meintanis S, Thomaidou D, Jessen KR, Mirsky R, Matsas R. (2004) Novel method for studying myelination in vivo reveals that EDTA is a potent inhibitor of myelin protein and mRNA expression during development of the rat sciatic nerve. Glia. 48:132-44.
Chapters in Collective Volumes
Thomaidou D, Politis PK and Matsas R (2010) Neurogenesis in the Central Nervous System: Cell Cycle Progression/Exit and Differentiation of Neuronal Progenitors. In: Cell Cycle Regulation and Differentiation in Cardiovascular and Neural Systems A. Giordano, U. Galderisi (eds.), Springer Science DOI 10.1007/978-1-60327-153-0_8.
Lavdas A and Matsas R (2009). Schwann cell morphology In: New Encyclopedia of Neuroscience (L.R. Squire, Editor) Oxford: Academic Press, pp 475-484.
Αφιέρωμα Βλαστοκύτταρα: ΕΛΕΥΘΕΡΟΣ ΤΥΠΟΣ Τετάρτη 9 Ιουλίου 2008, Παρουσίαση της Μονάδας Βλαστοκυττάρων του ΕΙΠ.
Αφιέρωμα ΒΗΜΑ Science, Κυριακή 29 Ιουλίου 2007 ΚΥΤΤΑΡΑ ΔΙΑΣΩΣΤΕΣ ΣΤΗ ΣΠΟΝΔΥΛΙΚΗ ΣΤΗΛΗ: Ελπίδες για θεραπεία των παραλυτικών κακώσεων της σπονδυλικής στήλης δίνει έρευνα σε πειραματόζωα που πραγματοποίησαν έλληνες ερευνητές του Ινστιτούτου Παστέρ. Ποντίκια κατάφεραν να χρησιμοποιήσουν ξανά τα πόδια τους, όταν κυτταρική «γέφυρα διάσωσης» επέτρεψε την αναγέννηση κατεστραμμένου νευρικού ιστού.
Αφιέρωμα «Οι πρωτοπόροι» Ε_ΙΣΤΟΡΙΚΑ ΕΛΕΥΘΕΡΟΤΥΠΙΑ 2006, Υπεύθυνος Έκδοσης: Π. Παναγόπουλος. Ρεβέκκα Μάτσα: Λουί Παστέρ: Η ζωή και το έργο του.
Αλέξανδρος Λάβδας και Ρεβέκκα Μάτσα: Λουί Παστέρ: Από το κρασί στη δημιουργία της ζωής.
Ρεβέκκα Μάτσα και Αλέξανδρος Λάβδας: Λουί Παστέρ: Τα πρώτα εμβόλια.
Αφιέρωμα Βλαστοκύτταρα: ΒΗΜΑ ΙΔΕΩΝ Τεύχος 04/7/2008 www.vimaideon.gr Ρεβέκκα Μάτσα «Θα μπορέσουμε να θεραπεύσουμε τις παθήσεις του εγκεφάλου;».
Αφιέρωμα «Οι πρωτοπόροι» Ε_ΙΣΤΟΡΙΚΑ ΕΛΕΥΘΕΡΟΤΥΠΙΑ 2006, Υπεύθυνος Έκδοσης: Π. Παναγόπουλος. Ρεβέκκα Μάτσα: Λουί Παστέρ: Η ζωή και το έργο του.



